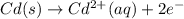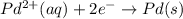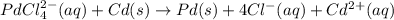Chemistry, 10.12.2019 06:31 antoinewill05
Avoltaic cell that uses the reaction pdcl2−4(aq)+cd(s)→pd(s)+4cl−(aq)+cd 2+(aq) has a measured standard cell potential of +1.03 v.
part a) write the two half-cell reactions. express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3


Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
Avoltaic cell that uses the reaction pdcl2−4(aq)+cd(s)→pd(s)+4cl−(aq)+cd 2+(aq) has a measured stand...
Questions



Mathematics, 09.10.2019 00:10








History, 09.10.2019 00:10