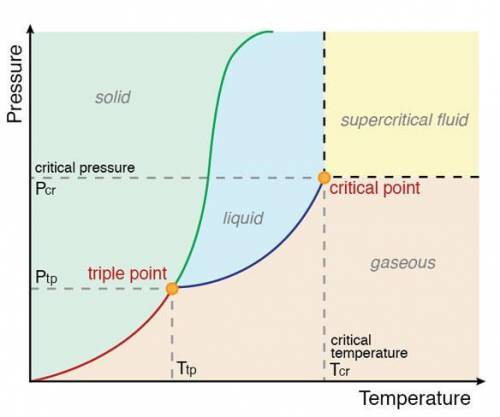
Acertain substance, x, has a triple-point temperature of 20°c at a pressure of 2.0 atm.
...

Chemistry, 10.12.2019 06:31 murphyscott794
Acertain substance, x, has a triple-point temperature of 20°c at a pressure of 2.0 atm.
which one of the following statements cannot possibly be true?
a) x can exist as a liquid above 20°c.
b) x can exist as a solid above 20°c.
c) liquid x can exist as a stable phase at 25°c, 1 atm.
d) both liquid and solid x have the same vapor pressure at 20°c.
e) all of these statements could be true

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2


Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Questions

Mathematics, 16.09.2019 19:00




History, 16.09.2019 19:00






Computers and Technology, 16.09.2019 19:00







Computers and Technology, 16.09.2019 19:00