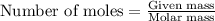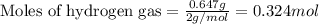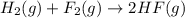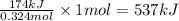The reaction of hydrogen(g) with fluorine(g) to form hydrogen fluoride(g) proceeds as follows: h2(g) + f2(g) 2 hf(g) when 0.647 grams of h2(g) react with sufficient f2(g) , 174 kj of energy are evolved . what is the value of h for the chemical equation given? δhrxn = kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1
You know the right answer?
The reaction of hydrogen(g) with fluorine(g) to form hydrogen fluoride(g) proceeds as follows: h2(g...
Questions


Mathematics, 24.11.2020 17:40


English, 24.11.2020 17:40

Mathematics, 24.11.2020 17:40


Chemistry, 24.11.2020 17:40



Arts, 24.11.2020 17:40

Business, 24.11.2020 17:40

Chemistry, 24.11.2020 17:50


Advanced Placement (AP), 24.11.2020 17:50





Mathematics, 24.11.2020 17:50

Mathematics, 24.11.2020 17:50

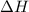 for the reaction will be -537 kJ
for the reaction will be -537 kJ