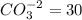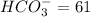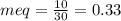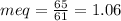Chemistry, 10.12.2019 05:31 DantesinfernoxD9964
1. a water with a ph of 9.0 contains 10 mg/l co3-2 and 65 mg/l hco3-. calculate the alkalinity of the water expressed as meq/l (milli equivalents per liter) and as mg/l as caco3.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2


Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1

Chemistry, 23.06.2019 10:00
An uncovered pot of water lies out in the sun. which statements correctly describe what happens at the surface of the liquid water? 1. the vapor pressure remains constant regardless of the water temperature. 2. the vapor pressure is produced by water molecules that have evaporated. 3. the vapor pressure increases as the sun heats the water in the pot. 4. evaporation stops once the vapor pressure reaches a certain point. 5. evaporation and condensation both occur on the liquid’s surface.
Answers: 3
You know the right answer?
1. a water with a ph of 9.0 contains 10 mg/l co3-2 and 65 mg/l hco3-. calculate the alkalinity of th...
Questions


Biology, 18.07.2019 04:00

Mathematics, 18.07.2019 04:00

Mathematics, 18.07.2019 04:00

Spanish, 18.07.2019 04:00

Mathematics, 18.07.2019 04:00



Social Studies, 18.07.2019 04:00

History, 18.07.2019 04:00




Mathematics, 18.07.2019 04:00


Mathematics, 18.07.2019 04:00

History, 18.07.2019 04:00


Mathematics, 18.07.2019 04:00

![\frac{mg}{L}[Carbonate]=0.33X50=16.5\frac{mg}{L} CaCO_{3}](/tpl/images/0411/3097/8200e.png)
![\frac{mg}{L}[BiCarbonate]=1.06X50=53\frac{mg}{L} CaCO_{3}](/tpl/images/0411/3097/a0838.png)