
Chemistry, 10.12.2019 05:31 kimberlyhansen92
Acertain liquid x has a normal boiling point of 108.30 °c and a boiling point elevation constant kb=1.07 °c kg/mol. a solution is prepared by dissolving some ammonium sulfate (nh4)2 so4 in 600. g of x. this solution boils at 109.7 °c. calculate the mass of (nh4)2 so4 that was dissolved. be sure your answer is rounded to the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Acertain liquid x has a normal boiling point of 108.30 °c and a boiling point elevation constant kb=...
Questions

Computers and Technology, 17.12.2020 23:10

Mathematics, 17.12.2020 23:10




Mathematics, 17.12.2020 23:10

Mathematics, 17.12.2020 23:20

Mathematics, 17.12.2020 23:20



Mathematics, 17.12.2020 23:20


Mathematics, 17.12.2020 23:20


Mathematics, 17.12.2020 23:20


Social Studies, 17.12.2020 23:20


Mathematics, 17.12.2020 23:20

Geography, 17.12.2020 23:20

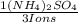 = 0,262 moles of (NH₄)₂SO₄
= 0,262 moles of (NH₄)₂SO₄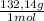 = 34,6g of (NH₄)₂SO₄
= 34,6g of (NH₄)₂SO₄

