
Chemistry, 10.12.2019 05:31 annabanana1298
1‑propanol ( n ‑propanol) and 2‑propanol (isopropanol) form ideal solutions in all proportions. calculate the partial pressure and the mole fraction ( y ) of the vapor phase of each component in equilibrium with each of the given solutions at 25 °c. p ∘ prop = 20.9 torr and p ∘ iso = 45.2 torr at 25 °c. a solution with a mole fraction of x prop = 0.247 .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Seawater contains approximately 3.5%nacl by mass and has a density of 1.02 g/ml. what volume of seawater contains 7.5 g of sodium?
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
1‑propanol ( n ‑propanol) and 2‑propanol (isopropanol) form ideal solutions in all proportions. calc...
Questions



Mathematics, 11.12.2020 04:10

English, 11.12.2020 04:10

Mathematics, 11.12.2020 04:10

Geography, 11.12.2020 04:10

Advanced Placement (AP), 11.12.2020 04:10




History, 11.12.2020 04:10

Mathematics, 11.12.2020 04:10

Mathematics, 11.12.2020 04:10


Health, 11.12.2020 04:10




Mathematics, 11.12.2020 04:10

Mathematics, 11.12.2020 04:10

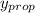 = 0.134;
= 0.134; 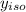 = 0.866
= 0.866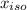 ) = 1 - mole fraction of propanol (
) = 1 - mole fraction of propanol (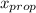 ).
). 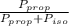
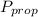 is the partial pressure of propanol and
is the partial pressure of propanol and 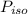 is the partial pressure of isopropanol.
is the partial pressure of isopropanol.

