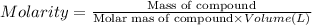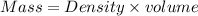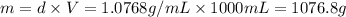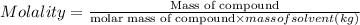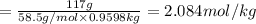Chemistry, 10.12.2019 05:31 hnsanders00
A2 m solution of nacl in water is at 20.0 oc. the density of the solution was measured to be 1.0768 g/ml at this temperature. calculate the molality of the salt solution. express your answer numerically to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

You know the right answer?
A2 m solution of nacl in water is at 20.0 oc. the density of the solution was measured to be 1.0768...
Questions


Medicine, 06.05.2020 08:03



Mathematics, 06.05.2020 08:03


Mathematics, 06.05.2020 08:03

Health, 06.05.2020 08:03

Geography, 06.05.2020 08:03


Mathematics, 06.05.2020 08:03

Mathematics, 06.05.2020 08:03

Mathematics, 06.05.2020 08:03

Mathematics, 06.05.2020 08:03

Mathematics, 06.05.2020 08:03




Mathematics, 06.05.2020 08:03

Chemistry, 06.05.2020 08:04