
Chemistry, 10.12.2019 04:31 monsterwins5001
Ammonia will decompose into nitrogen and hydrogen at high temperature. an industrial chemist studying this reaction fills a 500. ml flask with 2.3 atm of ammonia gas at 32. °c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 0.69 atm. calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. an industrial chemist studyin...
Questions

Arts, 26.09.2021 14:00



Mathematics, 26.09.2021 14:00


English, 26.09.2021 14:00

English, 26.09.2021 14:00





Mathematics, 26.09.2021 14:00





History, 26.09.2021 14:00

Mathematics, 26.09.2021 14:00


History, 26.09.2021 14:00

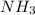 is as follows:.
is as follows:.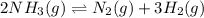
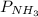 = 2.3 atm at equilibrium
= 2.3 atm at equilibrium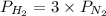 = 0.69 atm
= 0.69 atm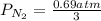
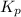 will be as follows.
will be as follows.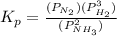
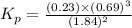
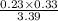
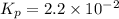
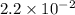 .
.


