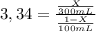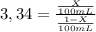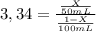Chemistry, 10.12.2019 04:31 lilianaalbarrap9tkqo
Achemist wants to extract a solute from 100 ml of water using only 300 ml of ether. the partition coefficient between ether and water is 3.34. calculate the fraction of solute that would remain in the water under each of the extraction conditions.
a. the chemist performs a single extraction with 300 ml of ether.
b. the chemist performs three extractions with 100 ml of ether.
c. the chemist performs six extractions with 50 ml of ether.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
Achemist wants to extract a solute from 100 ml of water using only 300 ml of ether. the partition co...
Questions

History, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00

Geography, 18.03.2021 01:00





Mathematics, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00



History, 18.03.2021 01:00

Computers and Technology, 18.03.2021 01:00



English, 18.03.2021 01:00



Mathematics, 18.03.2021 01:00

English, 18.03.2021 01:00