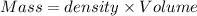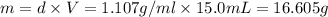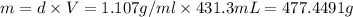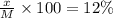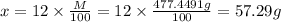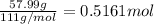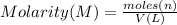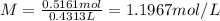A12.0 wt% solution of cacl2 (110.98 g/mol) has a density of 1.107 g/ml.
a) what is the mass (in milligrams) of a 15.0-ml solution of 12.0 wt% cacl2?
b) what is the mass (in grams) of cacl2 in 431.3 ml of a 12.0 wt% solution of cacl2?
c) what is the formal concentration of cacl2 (in molarity) of the 431.3-ml cacl2 solution described in part b?

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1
You know the right answer?
A12.0 wt% solution of cacl2 (110.98 g/mol) has a density of 1.107 g/ml.
a) what is the m...
a) what is the m...
Questions


History, 12.10.2019 18:10



History, 12.10.2019 18:10


Social Studies, 12.10.2019 18:10



Computers and Technology, 12.10.2019 18:10


Biology, 12.10.2019 18:10

Mathematics, 12.10.2019 18:10

Mathematics, 12.10.2019 18:10


Mathematics, 12.10.2019 18:10


History, 12.10.2019 18:10


Mathematics, 12.10.2019 18:10