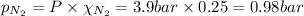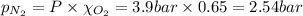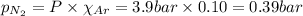Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Amixture of n2, o2, and ar has mole fractions of 0.25, 0.65, and 0.10, respectively. what is the pre...
Questions



History, 08.07.2019 14:30

World Languages, 08.07.2019 14:30

Physics, 08.07.2019 14:30














Health, 08.07.2019 14:30

History, 08.07.2019 14:30

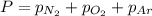
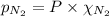
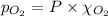
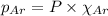
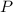 = total pressure = 3.9 bar
= total pressure = 3.9 bar  = partial pressure of nitrogen gas
= partial pressure of nitrogen gas 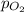 = partial pressure of oxygen gas
= partial pressure of oxygen gas 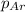 = partial pressure of argon gases
= partial pressure of argon gases 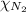 = Mole fraction of nitrogen gas = 0.25
= Mole fraction of nitrogen gas = 0.25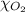 = Mole fraction of oxygen gas = 0.65
= Mole fraction of oxygen gas = 0.65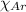 = Mole fraction of argon gases = 0.10
= Mole fraction of argon gases = 0.10