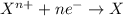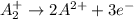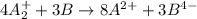Chemistry, 10.12.2019 04:31 summerhumphries3
Given two half reactions as follows: a2+ → 2 a2+ + 3 e− 4 e− + b → b4− what would you multiply each half-reaction by, to cancel out the electrons?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Given two half reactions as follows: a2+ → 2 a2+ + 3 e− 4 e− + b → b4− what would you multiply each...
Questions


Mathematics, 21.07.2019 02:20

History, 21.07.2019 02:20





History, 21.07.2019 02:20


Chemistry, 21.07.2019 02:20




Mathematics, 21.07.2019 02:20

Business, 21.07.2019 02:20



Mathematics, 21.07.2019 02:20


English, 21.07.2019 02:20