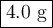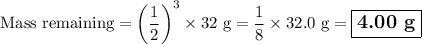Chemistry, 10.12.2019 02:31 angellll4455
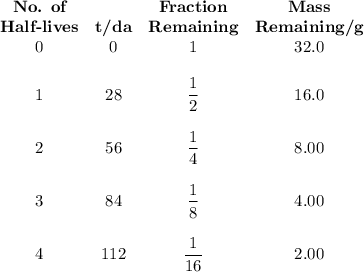
9.) the isotope chromium-51 has a half-life of 28 days. if the original sample had a 51cr mass of 32.0 grams, what mass of 51cr would remain after 84 days

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1


Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
9.) the isotope chromium-51 has a half-life of 28 days. if the original sample had a 51cr mass of 32...
Questions



Mathematics, 04.04.2020 17:40

Mathematics, 04.04.2020 17:40

Mathematics, 04.04.2020 17:40

History, 04.04.2020 17:40

Geography, 04.04.2020 17:40



Physics, 04.04.2020 17:41

Mathematics, 04.04.2020 17:41




English, 04.04.2020 17:41

English, 04.04.2020 17:41

Mathematics, 04.04.2020 17:41

Mathematics, 04.04.2020 17:41

History, 04.04.2020 17:41