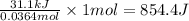Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3


Chemistry, 23.06.2019 09:20
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2
You know the right answer?
When 5.00 g of solid barium react with chlorine gas to produce solid barium chloride, 31.1 kj of hea...
Questions


Social Studies, 07.05.2021 19:00


Computers and Technology, 07.05.2021 19:00

Mathematics, 07.05.2021 19:00



History, 07.05.2021 19:00

Mathematics, 07.05.2021 19:00




Social Studies, 07.05.2021 19:10



Mathematics, 07.05.2021 19:10

Mathematics, 07.05.2021 19:10


English, 07.05.2021 19:10

Mathematics, 07.05.2021 19:10

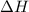 for the reaction will be -854.4 kJ
for the reaction will be -854.4 kJ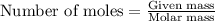
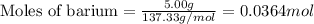
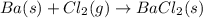
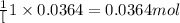 of barium chloride
of barium chloride