
Chemistry, 10.12.2019 01:31 bonetanne7171
Consider a solution containing 3.54 mm of an analyte, x, and 1.23 mm of a standard, s. upon chromatographic separation of the solution peak areas for x and s are 3275 and 10829, respectively. determine the response factor for x relative to s. f=to determine the concentration of x in an unknown solution, 1.00 ml of 8.27 mm s was added to 5.00 ml of the unknown x solution and the mixture was diluted to 10.0 ml. after chromatographic separation, this solution gave peak areas of 5389 and 4627 for x and s, respectively. determine the concentration of s in the 10.0 ml solution.[s]=determine the concentration of x in the 10.0 ml solution.[x]=determine the concentration of x in the unknown solution.[x]=

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
Consider a solution containing 3.54 mm of an analyte, x, and 1.23 mm of a standard, s. upon chromato...
Questions


Arts, 24.01.2021 18:40

English, 24.01.2021 18:40

Mathematics, 24.01.2021 18:40

English, 24.01.2021 18:40

Mathematics, 24.01.2021 18:40

Mathematics, 24.01.2021 18:40

Arts, 24.01.2021 18:40

English, 24.01.2021 18:40

Mathematics, 24.01.2021 18:40


Health, 24.01.2021 18:40


Mathematics, 24.01.2021 18:40


Computers and Technology, 24.01.2021 18:40




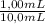 = 0,827mM
= 0,827mM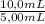 = 18,3mM
= 18,3mM

