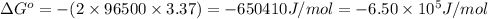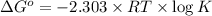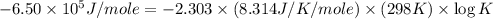Chemistry, 10.12.2019 01:31 adajadavis2843
Consider the following reaction. mg(s) + 2vo2+ (aq)+ 4 h+(aq) → mg2+(aq) + 2 vo2+(aq) + h2o(l)e⁰cell = 3.37 v. a.calculate the δg⁰∘ for the reaction. express your answer to three significant figures. b. calculate the k for the reaction. express your answer to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3
You know the right answer?
Consider the following reaction. mg(s) + 2vo2+ (aq)+ 4 h+(aq) → mg2+(aq) + 2 vo2+(aq) + h2o(l)e⁰cell...
Questions



Business, 28.08.2020 01:01

Mathematics, 28.08.2020 01:01

Chemistry, 28.08.2020 01:01

History, 28.08.2020 01:01


Mathematics, 28.08.2020 01:01

English, 28.08.2020 01:01

Mathematics, 28.08.2020 01:01

Mathematics, 28.08.2020 01:01

Mathematics, 28.08.2020 01:01


Geography, 28.08.2020 01:01

Mathematics, 28.08.2020 01:01

Mathematics, 28.08.2020 01:01


History, 28.08.2020 01:01

Physics, 28.08.2020 01:01

Advanced Placement (AP), 28.08.2020 01:01

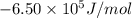
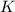 at 298 is,
at 298 is, 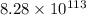
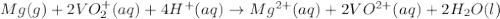
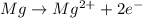
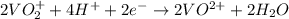
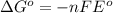
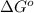 = Gibbs free energy = ?
= Gibbs free energy = ?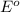 = standard e.m.f of cell = 3.37 V
= standard e.m.f of cell = 3.37 V