
Consider the reactiona + b → productsfrom the following data obtained at a certain temperature, determine the order of the reaction. enter the order with respect to a, the order with respect to b, and the overall reaction order.[a] (m) [b] (m) rate (m/s)1.50 1.50 3.20 ×10−11.50 2.50 3.20 ×10−13.00 1.50 6.40 ×10−1

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Consider the reactiona + b → productsfrom the following data obtained at a certain temperature, dete...
Questions









Mathematics, 13.10.2020 01:01


Geography, 13.10.2020 01:01


Mathematics, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01

Social Studies, 13.10.2020 01:01

Biology, 13.10.2020 01:01

English, 13.10.2020 01:01


Geography, 13.10.2020 01:01

![Rate=k[A]^x[B]^y](/tpl/images/0410/9200/ddde1.png)
![3.20\times 10^{-1}=k[1.50]^x[1.50]^y](/tpl/images/0410/9200/dd6b9.png) (1)
(1)
![3.20\times 10^{-1}=k[1.50]^x[2.50]^y](/tpl/images/0410/9200/0ba06.png) (2)
(2)
![\frac{3.20\times 10^{-1}}{3.20\times 10^{-1}}=\frac{k[1.50]^x[2.50]^y}{k[1.50]^x[1.50]^y}](/tpl/images/0410/9200/fa12d.png)
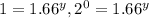 therefore y=0
therefore y=0![6.40\times 10^{-1}=k[3.00]^x[1.50]^y](/tpl/images/0410/9200/1edfc.png) (4)
(4)
![\frac{6.40\times 10^{-1}}{3.20\times 10^{-1}}=\frac{k[3.00]^x[1.50]^y}{k[1.50]^x[2.50]^y}](/tpl/images/0410/9200/10d3f.png)
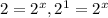 , x=1
, x=1
![Rate=k[A]^1[B]^0](/tpl/images/0410/9200/f8c47.png)


