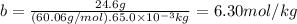Acertain substance condenses at a temperature of 123.3°c . but if a 65.0 gm sample of x is prepared with 24.6g of urea ((nh2)2co) dissolved in it, the sample is found to have a condensation point of 124.3°c instead.
1. calculate the molal boiling point elevation constant kb of x . round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
Acertain substance condenses at a temperature of 123.3°c . but if a 65.0 gm sample of x is prepared...
Questions

Mathematics, 28.09.2019 19:30

History, 28.09.2019 19:30



Computers and Technology, 28.09.2019 19:30

Computers and Technology, 28.09.2019 19:30

Chemistry, 28.09.2019 19:30

Mathematics, 28.09.2019 19:30




History, 28.09.2019 19:30

History, 28.09.2019 19:30

Mathematics, 28.09.2019 19:30

Computers and Technology, 28.09.2019 19:30

Spanish, 28.09.2019 19:30


Computers and Technology, 28.09.2019 19:30

Mathematics, 28.09.2019 19:30

Computers and Technology, 28.09.2019 19:30