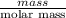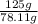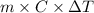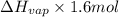Chemistry, 10.12.2019 00:31 elijahsantiago21
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k to liquify the sample and lower the temperature to 335.0 k? the following physical data may be useful.δhvap = 33.9 kj/molδhfus = 9.8 kj/molcliq = 1.73 j/g°ccgas = 1.06 j/g°ccsol = 1.51 j/g°ctmelting = 279.0 ktboiling = 353.0 k

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1
You know the right answer?
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k...
Questions


History, 31.08.2019 16:00


Mathematics, 31.08.2019 16:00



History, 31.08.2019 16:00

History, 31.08.2019 16:00




Mathematics, 31.08.2019 16:00

Mathematics, 31.08.2019 16:00


Mathematics, 31.08.2019 16:00

Social Studies, 31.08.2019 16:00

Mathematics, 31.08.2019 16:00

Geography, 31.08.2019 16:00

Social Studies, 31.08.2019 16:00

Biology, 31.08.2019 16:00