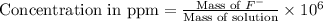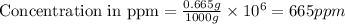Chemistry, 09.12.2019 23:31 camiloriveraveoxbgd6
Express the concentration of a 0.0350 m aqueous solution of fluoride, f − , in mass percentage and in parts per million (ppm). assume the density of the solution is 1.00 g/ml. a. number in percentage %
b. number in ppm .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3


Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1
You know the right answer?
Express the concentration of a 0.0350 m aqueous solution of fluoride, f − , in mass percentage and i...
Questions

History, 31.07.2019 23:00

English, 31.07.2019 23:00

History, 31.07.2019 23:00

Business, 31.07.2019 23:00

Mathematics, 31.07.2019 23:00


Mathematics, 31.07.2019 23:00

Mathematics, 31.07.2019 23:00

Geography, 31.07.2019 23:00


Biology, 31.07.2019 23:00


Biology, 31.07.2019 23:00

Mathematics, 31.07.2019 23:00



English, 31.07.2019 23:00

History, 31.07.2019 23:00


Mathematics, 31.07.2019 23:00

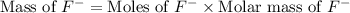
 = 19 g/mole
= 19 g/mole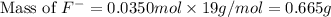
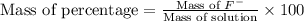
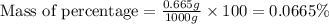
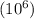 parts by the mass of the solution.
parts by the mass of the solution.