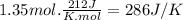Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
The normal boiling point of methanol is 64.7 ∘ c and the molar enthalpy of vaporization is 71.8kj/mo...
Questions


Advanced Placement (AP), 03.05.2021 19:20


Mathematics, 03.05.2021 19:20

History, 03.05.2021 19:20




Mathematics, 03.05.2021 19:20

Mathematics, 03.05.2021 19:20

Physics, 03.05.2021 19:20


Biology, 03.05.2021 19:20


Chemistry, 03.05.2021 19:20

Mathematics, 03.05.2021 19:20