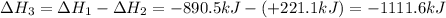Chemistry, 09.12.2019 22:31 santos200154
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 890.5 kj xco 3 ( s ) ⟶ xo ( s ) + co 2 ( g ) δ h = + 221.1 kj what is δ h for this reaction? x ( s ) + 1 2 o 2 ( g ) + co 2 ( g ) ⟶ xco 3 ( s )

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 890.5 kj xco 3 ( s ) ⟶ xo ( s ) + co...
Questions

Mathematics, 28.01.2021 04:30

Mathematics, 28.01.2021 04:30

Biology, 28.01.2021 04:30

Mathematics, 28.01.2021 04:30



Social Studies, 28.01.2021 04:30




Mathematics, 28.01.2021 04:30

Mathematics, 28.01.2021 04:30


History, 28.01.2021 04:30


Mathematics, 28.01.2021 04:30

English, 28.01.2021 04:30



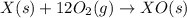
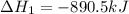 (1)
(1)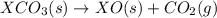
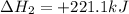 (2)
(2)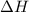 for the following reaction i.e,
for the following reaction i.e,
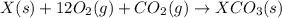
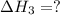 (3)
(3)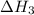 for the reaction will be:
for the reaction will be: