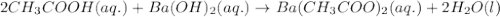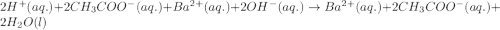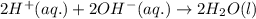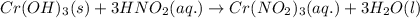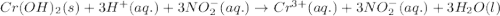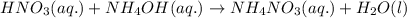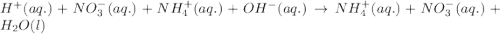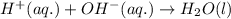Chemistry, 23.11.2019 09:31 sepdentalcare8774
Write the balanced molecular and net ionic equations for each of the following neutralization reactions. (a) aqueous acetic acid (hc2h3o2) is neutralized by aqueous barium hydroxide. (b) solid chromium(iii) hydroxide reacts with nitrous acid. (c) aqueous nitric acid and aqueous ammonia react.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

You know the right answer?
Write the balanced molecular and net ionic equations for each of the following neutralization reacti...
Questions


Physics, 21.04.2020 03:14





History, 21.04.2020 03:14


Mathematics, 21.04.2020 03:14

History, 21.04.2020 03:14




Mathematics, 21.04.2020 03:14

Mathematics, 21.04.2020 03:14

History, 21.04.2020 03:14




English, 21.04.2020 03:15

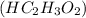 is neutralized by aqueous barium hydroxide.
is neutralized by aqueous barium hydroxide.