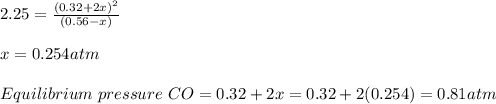Consider the following reaction:
co2(g) + c(graphite) ⇌ 2 co(g)
a reaction...

Chemistry, 22.11.2019 19:31 laurenbreellamerritt
Consider the following reaction:
co2(g) + c(graphite) ⇌ 2 co(g)
a reaction mixture initially contains 0.56 atm co2 and 0.32 atm co. determine the equilibrium pressure of co if kp for the reaction at this temperature is 2.25.
a) 0.83 atm
b) 0.31 atm
c) 0.26 atm
d) 0.58 atm
e) 0.42 atm

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Strong conductivity of plasma allows it to act and react as and
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
Questions


Mathematics, 01.02.2021 21:50


Mathematics, 01.02.2021 21:50

Spanish, 01.02.2021 21:50


Mathematics, 01.02.2021 21:50


Advanced Placement (AP), 01.02.2021 21:50




English, 01.02.2021 21:50

Mathematics, 01.02.2021 21:50




Mathematics, 01.02.2021 21:50


![\frac{ [0.32+x]^{2} }{ 0.56-x_{2} }](/tpl/images/0386/6848/7ac95.png) where x is the amount that is produced. x s calculated is 0.255 atm. Thus, the final partial pressure of CO is 0.83 atm. Answer is A.
where x is the amount that is produced. x s calculated is 0.255 atm. Thus, the final partial pressure of CO is 0.83 atm. Answer is A.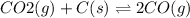
![Kp = \frac{[CO]^{2} }{[CO2]}](/tpl/images/0386/6848/00175.png)