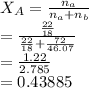Chemistry, 09.12.2019 19:31 historyfanboy101
The vapor pressure of pure water at 15 °c is 12.8 mm hg. what is the equilibrium vapor pressure of water above a mixture of 72.0 g ethanol (ch3ch2oh, molar mass = 46.07 g/mol) and 22.0 g water?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1


Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1
You know the right answer?
The vapor pressure of pure water at 15 °c is 12.8 mm hg. what is the equilibrium vapor pressure of w...
Questions

Mathematics, 20.10.2020 14:01

Physics, 20.10.2020 14:01

History, 20.10.2020 14:01

Mathematics, 20.10.2020 14:01


Physics, 20.10.2020 14:01

Computers and Technology, 20.10.2020 14:01

Mathematics, 20.10.2020 14:01


Mathematics, 20.10.2020 14:01

Mathematics, 20.10.2020 14:01


English, 20.10.2020 14:01

English, 20.10.2020 14:01


History, 20.10.2020 14:01

Mathematics, 20.10.2020 14:01

English, 20.10.2020 14:01

Biology, 20.10.2020 14:01

English, 20.10.2020 14:01

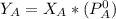
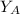 is the partial vapour pressure ( mm Hg)
is the partial vapour pressure ( mm Hg)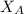 is the mole fraction
is the mole fraction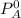 is the pure vapour pressure = 12.8 mm Hg
is the pure vapour pressure = 12.8 mm Hg