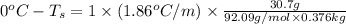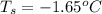Chemistry, 09.12.2019 19:31 nayelimoormann
Determine the freezing point depression of a solution that contains 30.7 g glycerin (c3h8o3, molar mass = 92.09 g/mol) in 376 ml of water. some possibly useful constants for water are kf = 1.86°c/m and kb = 0.512°c/m.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
Determine the freezing point depression of a solution that contains 30.7 g glycerin (c3h8o3, molar m...
Questions


Advanced Placement (AP), 05.05.2020 22:31



Mathematics, 05.05.2020 22:31


Mathematics, 05.05.2020 22:31

Spanish, 05.05.2020 22:31

English, 05.05.2020 22:31


English, 05.05.2020 22:31

Mathematics, 05.05.2020 22:31

Mathematics, 05.05.2020 22:31




Mathematics, 05.05.2020 22:31

Business, 05.05.2020 22:31


History, 05.05.2020 22:31

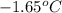
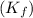 for water =
for water = 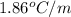

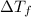 = change in freezing point
= change in freezing point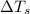 = freezing point of solution = ?
= freezing point of solution = ?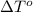 = freezing point of water =
= freezing point of water = 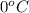
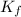 = freezing point constant for water =
= freezing point constant for water =