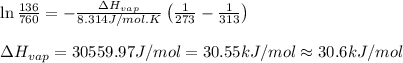Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
The vapor pressure of dichloromethane, ch2cl2, at 0 ∘c is 134 mmhg. the normal boiling point of dich...
Questions





World Languages, 21.10.2020 19:01



Mathematics, 21.10.2020 19:01


Mathematics, 21.10.2020 19:01



English, 21.10.2020 19:01

English, 21.10.2020 19:01

Biology, 21.10.2020 19:01





German, 21.10.2020 19:01

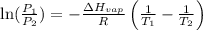
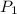 = vapor pressure at temperature
= vapor pressure at temperature 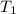 = 134 mmHg
= 134 mmHg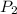 = vapor pressure at temperature
= vapor pressure at temperature 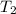 (atmospheric pressure) = 760 mmHg
(atmospheric pressure) = 760 mmHg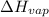 = molar heat of vaporization
= molar heat of vaporization![0^oC=[273+0]K=273K](/tpl/images/0410/2746/614a3.png)
![40^oC=[273+40]K=313K](/tpl/images/0410/2746/c9054.png)