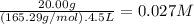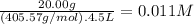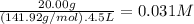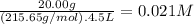Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3


Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
What is the molarity of each ion present in aqueous solutions prepared by dissolving 20.00 g of the...
Questions

Mathematics, 30.12.2019 11:31

Mathematics, 30.12.2019 11:31

Physics, 30.12.2019 11:31




Mathematics, 30.12.2019 11:31






English, 30.12.2019 11:31

English, 30.12.2019 11:31


Mathematics, 30.12.2019 11:31

Business, 30.12.2019 11:31

Business, 30.12.2019 11:31