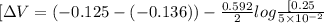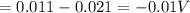Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 13:30
What is matter? a. anything that has mass and takes up space b. something that has volume and takes up space. c. things that have energy and take up space d. things that take up space but don't have mass
Answers: 2

Chemistry, 23.06.2019 16:30
In chile, the deepest earthquake occurred at 61.7°w longitude at a depth of 540 km. if the rocks at the focus began subducting 10 million years ago and are now 1000 km from their original position, what is the average rate of subduction in cm/yr?
Answers: 1
You know the right answer?
(a) compute the voltage at 25°c of an electrochemical cell consisting of pure lead immersed in a 5 ×...
Questions

Mathematics, 20.04.2021 19:30



Chemistry, 20.04.2021 19:30

English, 20.04.2021 19:30

World Languages, 20.04.2021 19:30


Mathematics, 20.04.2021 19:30

Mathematics, 20.04.2021 19:30


Mathematics, 20.04.2021 19:30

English, 20.04.2021 19:30





Mathematics, 20.04.2021 19:30

Mathematics, 20.04.2021 19:30


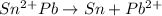
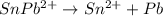
![[\Delta V=(V_{pb}^{0}-V_{sn}^{0})-\frac{0.592}{n}log\frac{[Sn^{2+}]}{[Pb^{2+}]}](/tpl/images/0410/3129/9e221.png)
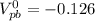
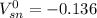
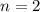
![[Sn^{2+}]= 0.25M](/tpl/images/0410/3129/b50c0.png)
![[Pb^{2+}]=5\times 10^{-2}M](/tpl/images/0410/3129/01669.png)