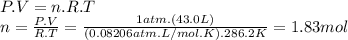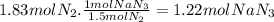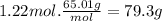The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of sodium azide, which produces large volumes of nitrogen gas. 1. write a balanced chemical equation, including physical state symbols, for the decomposition of solid sodium azide ( nan3 ) into solid sodium and gaseous dinitrogen. 2. suppose 43.0l of dinitrogen gas are produced by this reaction, at a temperature of 13.0°c and pressure of exactly 1atm . calculate the mass of sodium azide that must have reacted. round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of...
Questions


Chemistry, 01.03.2021 23:40


History, 01.03.2021 23:40




Mathematics, 01.03.2021 23:40




Chemistry, 01.03.2021 23:40


Mathematics, 01.03.2021 23:40

Chemistry, 01.03.2021 23:40




Computers and Technology, 01.03.2021 23:40

Mathematics, 01.03.2021 23:40