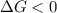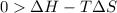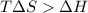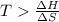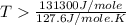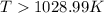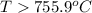Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3


Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2
You know the right answer?
For the reaction c(s)+h2o(g)→co2(g)+h2(g) δh∘=131.3kj/mol and δs∘=127.6j/k⋅mol at 298k. at temperatu...
Questions








Engineering, 05.09.2020 08:01





Spanish, 05.09.2020 08:01

Mathematics, 05.09.2020 08:01


Advanced Placement (AP), 05.09.2020 08:01



English, 05.09.2020 08:01

Mathematics, 05.09.2020 08:01

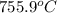 this reaction is spontaneous under standard conditions.
this reaction is spontaneous under standard conditions.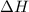 = 131.3 KJ/mole = 131300 J/mole
= 131.3 KJ/mole = 131300 J/mole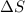 = 127.6 J/mole.K
= 127.6 J/mole.K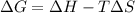
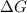 is negative or we can say that the value of
is negative or we can say that the value of