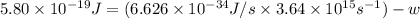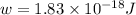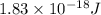Chemistry, 09.12.2019 18:31 gajdmaciej9502
When a metal was exposed to light at a frequency of 3.64× 1015 s–1, electrons were emitted with a kinetic energy of 5.80× 10–19 j. what is the maximum number of electrons that could be ejected from this metal by a burst of light (at some other frequency) with a total energy of 8.66× 10–7 j?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

You know the right answer?
When a metal was exposed to light at a frequency of 3.64× 1015 s–1, electrons were emitted with a ki...
Questions

History, 22.07.2019 18:30

Mathematics, 22.07.2019 18:30



Mathematics, 22.07.2019 18:30

History, 22.07.2019 18:30



Mathematics, 22.07.2019 18:30



Geography, 22.07.2019 18:30

Mathematics, 22.07.2019 18:30







Mathematics, 22.07.2019 18:30

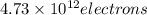
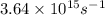
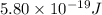
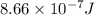
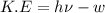
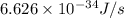
 = frequency
= frequency