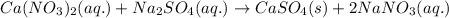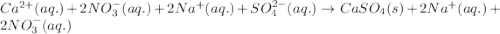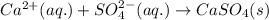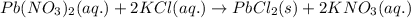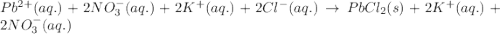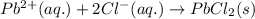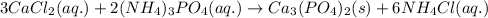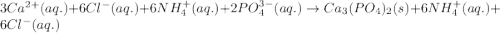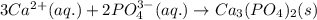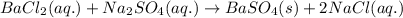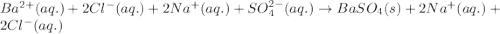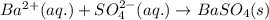Chemistry, 09.12.2019 17:31 jbehrens6538
Write the net ionic equation to show the formation of a solid (insoluble salt) when the following solutions are mixed. write noreaction if there is no precipitate. express your answer as a chemical equation. identify all of the phases in your answer. enter noreaction if no precipitate is formed.1. ca(no3)2(aq) + na2so4(aq)2. kcl(aq) + pb(no3)2(aq)3. cacl2(aq) + (nh4)3(po4)(aq)4. na2so4(aq) + bacl2(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
Write the net ionic equation to show the formation of a solid (insoluble salt) when the following so...
Questions


Mathematics, 10.12.2020 18:00




Mathematics, 10.12.2020 18:00

Mathematics, 10.12.2020 18:00


Law, 10.12.2020 18:00



History, 10.12.2020 18:00






Engineering, 10.12.2020 18:00