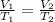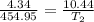Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
Asample of gas at 181.8 ◦c has a volume of 4.34 l. the volume expands to 10.44 l due to a temperatur...
Questions


History, 22.01.2021 22:20

Arts, 22.01.2021 22:20

Mathematics, 22.01.2021 22:20


Mathematics, 22.01.2021 22:20




Mathematics, 22.01.2021 22:20


Mathematics, 22.01.2021 22:20



English, 22.01.2021 22:20

English, 22.01.2021 22:20

Chemistry, 22.01.2021 22:20

History, 22.01.2021 22:20


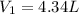
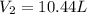
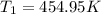
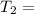 unknown
unknown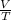 = constant
= constant