
Chemistry, 01.12.2019 00:31 deaishaajennings123
Calculate the approximate enthalpy change, δhrxn, for the combustion of methane: ch4+2o2→2h2o+co2 δhrxn from a given table: ch4 = 1656 kj/mol o2 = 498 kj/mol h2o = 928 kj/mol co2 = 1598 kj/mol?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 09:10
In a 28 g serving of cheese curls there are 247mg of sodium. how much sodium is in a 12.5 ounce bag
Answers: 1
You know the right answer?
Calculate the approximate enthalpy change, δhrxn, for the combustion of methane: ch4+2o2→2h2o+co2 δ...
Questions



Social Studies, 03.02.2020 22:47




History, 03.02.2020 22:47

Mathematics, 03.02.2020 22:47


Mathematics, 03.02.2020 22:47



Mathematics, 03.02.2020 22:47

Physics, 03.02.2020 22:47

Mathematics, 03.02.2020 22:47

Mathematics, 03.02.2020 22:47


Mathematics, 03.02.2020 22:47


Physics, 03.02.2020 22:47

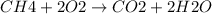
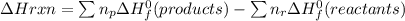
![\Delta Hrxn = [2\Delta H_{f}^{0}(H2O)+1\Delta H_{f}^{0}(CO2)]-[1\Delta H_{f}^{0}(CH4)+ 2\Delta H_{f}^{0}(O2)]](/tpl/images/0397/7957/b0337.png)


