
Chemistry, 07.12.2019 06:31 emmarieasimon
Acertain first-order reaction (a→products) has a rate constant of 7.20×10−3 s−1 at 45 ∘c. how many minutes does it take for the concentration of the reactant, [a], to drop to 6.25% of the original concentration?
express your answer with the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Acertain first-order reaction (a→products) has a rate constant of 7.20×10−3 s−1 at 45 ∘c. how many m...
Questions



Mathematics, 14.11.2019 05:31

Mathematics, 14.11.2019 05:31




History, 14.11.2019 05:31


English, 14.11.2019 05:31



English, 14.11.2019 05:31

Mathematics, 14.11.2019 05:31

History, 14.11.2019 05:31

Mathematics, 14.11.2019 05:31




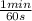 = 6,41 min
= 6,41 min

