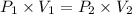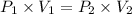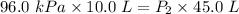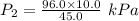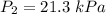Acylinder is filled with 10.0l of gas and a piston is put into it. the initial pressure of the gas is measured to be 96.0kpa. the piston is now pulled up, expanding the gas, until the gas has a final volume of 45.0l. calculate the final pressure of the gas. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
Acylinder is filled with 10.0l of gas and a piston is put into it. the initial pressure of the gas i...
Questions

English, 28.01.2021 02:50

Advanced Placement (AP), 28.01.2021 02:50

English, 28.01.2021 02:50



World Languages, 28.01.2021 02:50







Advanced Placement (AP), 28.01.2021 02:50


Business, 28.01.2021 02:50


Mathematics, 28.01.2021 02:50



Mathematics, 28.01.2021 02:50