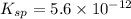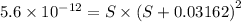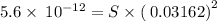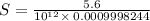Chemistry, 07.12.2019 05:31 NeverEndingCycle
What is the molar solubility of mg(oh)2 in a basic solution with a ph of 12.50? ksp for mg(oh)2 is 5.6 × 10-12. what is the molar solubility of mg(oh)2 in a basic solution with a ph of 12.50? ksp for mg(oh)2 is 5.6 × 10-12. 1.1 × 10-4 m 5.6 × 10-9 m 2.4 × 10-6 m 1.8 × 10-10 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
What is the molar solubility of mg(oh)2 in a basic solution with a ph of 12.50? ksp for mg(oh)2 is...
Questions

Health, 26.11.2019 22:31



Mathematics, 26.11.2019 22:31


History, 26.11.2019 22:31



History, 26.11.2019 22:31

Social Studies, 26.11.2019 22:31

Health, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31

History, 26.11.2019 22:31

Business, 26.11.2019 22:31


Mathematics, 26.11.2019 22:31

Biology, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31

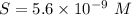
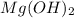 will form its respective ions in the solution as:
will form its respective ions in the solution as:
![K_{sp}=[Mg^{2+}][OH^-]^2](/tpl/images/0407/6768/48330.png)
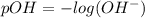
![[OH^-]=10^{(-1.5)}=0.03162](/tpl/images/0407/6768/a8c86.png)