
Chemistry, 07.12.2019 04:31 maxy7347go
In which solution(s) will a precipitate form? i. 0.00090 g of na2cro4 (molar mass = 162.0 g/mol) is added to 225 ml of 0.00025 m agno3. ksp ag2cro4 =1.1 x 10-12 ii. 0.100l of 0.0015 m mgcl2 and 0.200l of 0.012 m naf. ksp mgf2 = 3.7 x 10-8

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
The concept of empiricism states that all rationally accepted knowledge is determined from experience. francis bacon was one of the first scientists to promote this theory. what was it’s impact on society?
Answers: 1

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

You know the right answer?
In which solution(s) will a precipitate form? i. 0.00090 g of na2cro4 (molar mass = 162.0 g/mol) is...
Questions



Mathematics, 08.06.2021 01:30


Mathematics, 08.06.2021 01:30

Mathematics, 08.06.2021 01:30


Health, 08.06.2021 01:30

English, 08.06.2021 01:30


Mathematics, 08.06.2021 01:30






Mathematics, 08.06.2021 01:30



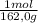 = 5,56x10⁻⁶ moles of Na₂CrO₄≡ moles of CrO₄²⁻.
= 5,56x10⁻⁶ moles of Na₂CrO₄≡ moles of CrO₄²⁻.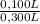 = 0,0005M MgCl₂≡ Mg²⁺
= 0,0005M MgCl₂≡ Mg²⁺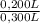 = 0,008M NaF ≡ F⁻
= 0,008M NaF ≡ F⁻

