
Chemistry, 07.12.2019 03:31 brainBoy480
Arock contains 0.275 mg of lead-206 for each milligram of uranium-238. the for the decay of uranium-238 to lead-206 is 4.5 x 10 9 yr. the rock was formed yr ago.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1


Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Arock contains 0.275 mg of lead-206 for each milligram of uranium-238. the for the decay of uranium-...
Questions

English, 19.05.2020 14:15

Arts, 19.05.2020 14:15


English, 19.05.2020 14:15



Physics, 19.05.2020 14:15


Chemistry, 19.05.2020 14:15






Mathematics, 19.05.2020 14:15



Mathematics, 19.05.2020 14:15


 years ago.
years ago. years
years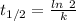
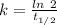
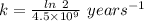
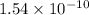 years⁻¹
years⁻¹![[A_t]=[A_0]e^{-kt}](/tpl/images/0407/4769/1ef89.png)
![[A_t]](/tpl/images/0407/4769/5262c.png) is the final concentration= 0.275 mg
is the final concentration= 0.275 mg![[A_0]](/tpl/images/0407/4769/9a686.png) is the initial concentration = 1 mg
is the initial concentration = 1 mg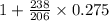 mg = 1.297 mg
mg = 1.297 mg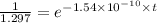
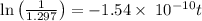
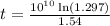
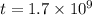 years
years


