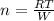Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
An experimenter adds 979 j of heat to an ideal gas to heat it from 10.6 c to 26.4 c at constant pres...
Questions

Health, 25.06.2019 11:30




Computers and Technology, 25.06.2019 11:30


Mathematics, 25.06.2019 11:30


Mathematics, 25.06.2019 11:30

Mathematics, 25.06.2019 11:30






Mathematics, 25.06.2019 11:30

Mathematics, 25.06.2019 11:30


Mathematics, 25.06.2019 11:30