
Chemistry, 07.12.2019 03:31 cbender30p860we
Calculate the change in entropy of the system when 10g of ice at -10c is converted into water vapor at 115c and at a constant pressure of 1bar. the constant pressure molar heat capacity of h2o(s) and h2o(l) is 75.291j/kmol and that of h2o(g) is 33.58j/kmo

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
Calculate the change in entropy of the system when 10g of ice at -10c is converted into water vapor...
Questions


Mathematics, 26.06.2019 07:50

Mathematics, 26.06.2019 07:50

Biology, 26.06.2019 07:50

History, 26.06.2019 07:50


Biology, 26.06.2019 07:50

Mathematics, 26.06.2019 07:50

Mathematics, 26.06.2019 07:50

Biology, 26.06.2019 07:50

Mathematics, 26.06.2019 07:50


Mathematics, 26.06.2019 07:50



Mathematics, 26.06.2019 07:50

History, 26.06.2019 07:50



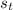 = Δ
= Δ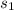 +Δ
+Δ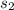 +Δ
+Δ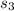 +Δ
+Δ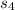
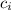 *
*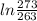
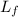 /273
/273 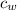 *
*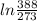
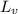 /388
/388 

