Chemistry, 07.12.2019 03:31 rbeltran24
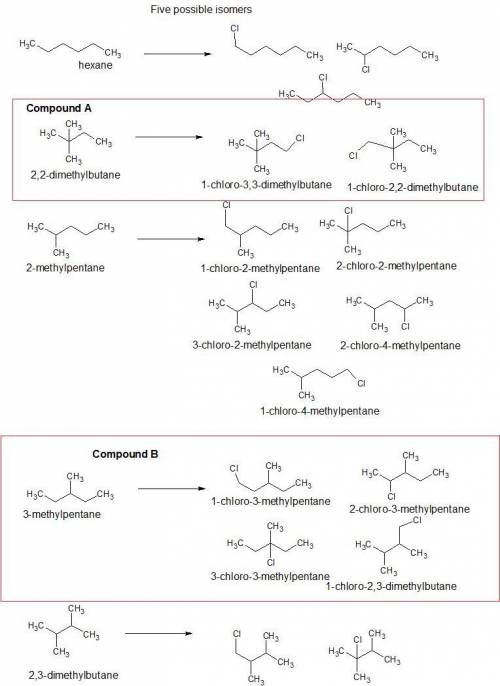
Both compounds a and b have molecular formula c6h14. monochlorination of compound a results in the formation of two constitutional isomers. monochlorination of compound b results in the formation of four constitutional isomers. identify compounds a and b, and show the products of each monochlorination.
1) draw compound a and the products of its monochlorination.
2)draw compound b and the products of its monochlorination.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2


Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
Both compounds a and b have molecular formula c6h14. monochlorination of compound a results in the f...
Questions

Biology, 19.07.2019 11:30

Biology, 19.07.2019 11:30


Social Studies, 19.07.2019 11:30

History, 19.07.2019 11:30

Biology, 19.07.2019 11:30

Social Studies, 19.07.2019 11:30

Biology, 19.07.2019 11:30

Biology, 19.07.2019 11:30

Biology, 19.07.2019 11:30

Social Studies, 19.07.2019 11:30

Mathematics, 19.07.2019 11:30

History, 19.07.2019 11:30

Biology, 19.07.2019 11:30

Biology, 19.07.2019 11:30

Social Studies, 19.07.2019 11:30

History, 19.07.2019 11:30

Biology, 19.07.2019 11:30

History, 19.07.2019 11:30

Social Studies, 19.07.2019 11:30