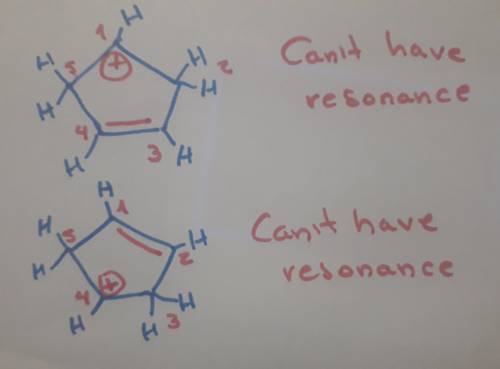
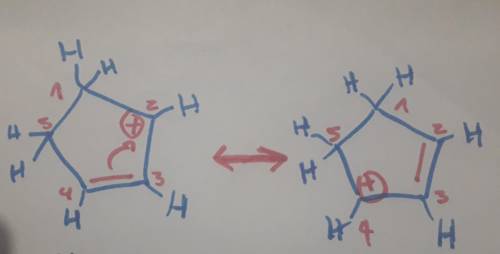
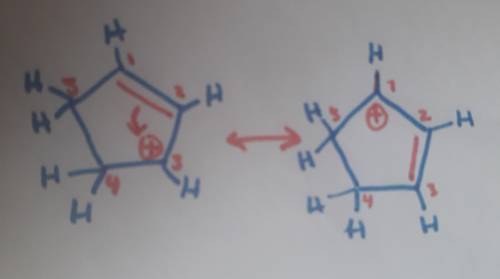
Carbons 1 and 4 of 1,3−cyclopentadiene are equivalent and give the same carbocation on protonation. likewise, carbons 2 and 3 are equivalent. write the structure of the carbocation formed by protonation of c−2 or c−3 to verify that it is not allylic and therefore not as stable as the one formed by protonation of c−1 or c−4.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

You know the right answer?
Carbons 1 and 4 of 1,3−cyclopentadiene are equivalent and give the same carbocation on protonation....
Questions





Biology, 19.08.2019 07:00




Mathematics, 19.08.2019 07:00

Chemistry, 19.08.2019 07:00

Mathematics, 19.08.2019 07:00

Geography, 19.08.2019 07:00



Geography, 19.08.2019 07:00

Physics, 19.08.2019 07:00


History, 19.08.2019 07:00

English, 19.08.2019 07:00