Consider a voltaic cell based on the following cell reaction:
ni(s) + at2(s) ? ni2+(aq) + 2a...

Chemistry, 07.12.2019 02:31 Matildagann
Consider a voltaic cell based on the following cell reaction:
ni(s) + at2(s) ? ni2+(aq) + 2at– (aq)
given that the standard cell emf is 0.55 v, what is the standard reduction potential for astatine? [e°(ni2+/ni) = –0.25 v]

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2
You know the right answer?
Questions

Social Studies, 20.11.2020 20:40

English, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40

Biology, 20.11.2020 20:40


History, 20.11.2020 20:40




English, 20.11.2020 20:40




Geography, 20.11.2020 20:40

Physics, 20.11.2020 20:40


Mathematics, 20.11.2020 20:40


![E^0_{[At_2/At^-]}=0.30V](/tpl/images/0407/4047/b7e3b.png)
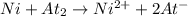
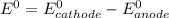
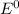 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Ni^{2+}/Mg]}= -0.25V](/tpl/images/0407/4047/bfc73.png)
![E^0_{[At_2/At^-]}=?](/tpl/images/0407/4047/cb54f.png)
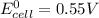
![E^0=E^0_{[At_2/At^-]}- E^0_{[Ni^{2+}/Mg]}](/tpl/images/0407/4047/e55a1.png)
![0.55V=E^0_{[At_2/At^-]-(-0.25V)](/tpl/images/0407/4047/9044a.png)


