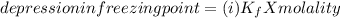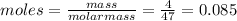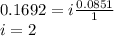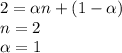Chemistry, 07.12.2019 02:31 kenzieeee96
When hno2 is dissolved in water it partially dissociates according to the equation hno2⇌h++no−2. a solution is prepared that contains 4.000 g of hno2 in 1.000 kg of water. its freezing point is found to be -0.1692 ∘c calculate the fraction of hno2 that has dissociated.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
When hno2 is dissolved in water it partially dissociates according to the equation hno2⇌h++no−2. a s...
Questions



Mathematics, 10.06.2021 21:20

Mathematics, 10.06.2021 21:20

English, 10.06.2021 21:20

History, 10.06.2021 21:20

English, 10.06.2021 21:20

Mathematics, 10.06.2021 21:20



Computers and Technology, 10.06.2021 21:20


Mathematics, 10.06.2021 21:20

Mathematics, 10.06.2021 21:20




Mathematics, 10.06.2021 21:20


Computers and Technology, 10.06.2021 21:20