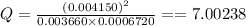Chemistry, 07.12.2019 02:31 aprilkenedy12
Consider this equilibrium reaction at 400 k. br2(g)+cl2(g)↽−−⇀2brcl(g)kc=7.0 br 2 ( g ) + cl 2 ( g ) ↽ − − ⇀ 2 brcl ( g ) k c = 7.0 if the composition of the reaction mixture at 400 k is [brcl]=0.004150 [ brcl ] = 0.004150 m, [br2]=0.003660 [ br 2 ] = 0.003660 m, and [cl2]=0.0006720 [ cl 2 ] = 0.0006720 m, what is the reaction quotient, q ?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Consider this equilibrium reaction at 400 k. br2(g)+cl2(g)↽−−⇀2brcl(g)kc=7.0 br 2 ( g ) + cl 2 ( g )...
Questions

Chemistry, 20.09.2020 08:01

English, 20.09.2020 08:01



Mathematics, 20.09.2020 08:01


Biology, 20.09.2020 08:01




Mathematics, 20.09.2020 08:01

History, 20.09.2020 08:01

Mathematics, 20.09.2020 08:01



English, 20.09.2020 08:01

Physics, 20.09.2020 08:01



History, 20.09.2020 08:01


![Q=\frac{[BrCl]^2}{[Br_2][Cl_2]}](/tpl/images/0407/4151/a2d2e.png)
![[BrCl]=0.004150\ M](/tpl/images/0407/4151/33a25.png)
![[Br_2]=0.003660\ M](/tpl/images/0407/4151/93561.png)
![[Cl_2}]=0.0006720\ M](/tpl/images/0407/4151/b53a5.png)