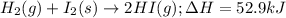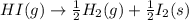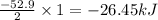Chemistry, 07.12.2019 00:31 hannahs1313
Given the following reaction: h2(g)+i2(s) → 2hi(g) with a ∆h of 52.9 kj. what is the change in enthalpy for the following reaction: hi(g) → 1 2h2(g)+1 2 i2(s)? express your answer in kj.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 11:50
Achemist needs to prepare a buffer solution of ph 8.80. what molarity of nh3 (pkb = 4.75) is required to produce the buffer solution if the (nh4)2so4 in the solution is 1.8 m?
Answers: 1
You know the right answer?
Given the following reaction: h2(g)+i2(s) → 2hi(g) with a ∆h of 52.9 kj. what is the change in enth...
Questions

English, 20.09.2020 15:01

Mathematics, 20.09.2020 15:01





Mathematics, 20.09.2020 15:01


Mathematics, 20.09.2020 15:01

World Languages, 20.09.2020 15:01


Mathematics, 20.09.2020 15:01





History, 20.09.2020 15:01

Mathematics, 20.09.2020 15:01


Mathematics, 20.09.2020 15:01