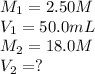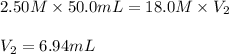Chemistry, 07.12.2019 00:31 juniorvaldez60
In a school’s laboratory, students require 50.0 ml of 2.50 m h2so4 for an experiment, but the only available stock solution of the acid has a concentration of 18.0 m. what volume of the stock solution would they use to make the required solutiona. 0.900 ml
b. 1.11 ml
c. 6.94 ml
d. 7.20 ml

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3


Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
In a school’s laboratory, students require 50.0 ml of 2.50 m h2so4 for an experiment, but the only a...
Questions




Chemistry, 28.05.2021 01:00

Spanish, 28.05.2021 01:00










Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00


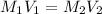
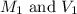 are the molarity and volume of acid which is
are the molarity and volume of acid which is 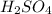
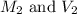 are the molarity and volume of stock solution of acid.
are the molarity and volume of stock solution of acid.