

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

You know the right answer?
In order to produce sp3d hybrid orbitals, s atomic orbital(s), p atomic orbital(s), and d atomic orb...
Questions






History, 28.05.2021 18:50

Biology, 28.05.2021 18:50

Mathematics, 28.05.2021 18:50

Mathematics, 28.05.2021 18:50

English, 28.05.2021 18:50


English, 28.05.2021 18:50

Mathematics, 28.05.2021 18:50





Chemistry, 28.05.2021 18:50


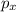 ,
, 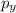 and
and 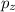 are half filled.
are half filled.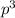 d hybrid orbitals, 1 s, 3 p and 1 d orbitals of approximately the same energy intermix are used to create 5 identical/degenerate hybrid orbitals. These orbitals are arranged to form a trigonal bipyramidal symmetry. Three orbitals are arranged in a trigonal plane and the other two orbitals are arranged up/down the trigonal plane at right angles.
d hybrid orbitals, 1 s, 3 p and 1 d orbitals of approximately the same energy intermix are used to create 5 identical/degenerate hybrid orbitals. These orbitals are arranged to form a trigonal bipyramidal symmetry. Three orbitals are arranged in a trigonal plane and the other two orbitals are arranged up/down the trigonal plane at right angles. 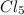 as an example, its formation requires five unpaired electron, the phosphorus atom was excited and one electron from the 3s filled one of the vacant 3d orbitals. Thus intermixing the orbitals created the s
as an example, its formation requires five unpaired electron, the phosphorus atom was excited and one electron from the 3s filled one of the vacant 3d orbitals. Thus intermixing the orbitals created the s

